At the Vrije Universiteit Brussel (VUB) – Flanders Institute for Biotechnology (VIB), biologist Jessie Vandierendonck has been investigating new, alternative treatments to combat bacterial infections using (bacterio)phages, viruses that attack and destroy bacteria.
“Bacteriophages were discovered even before the first antibiotic, and today we are once again recognising their enormous potential in the fight against antibiotic-resistant bacteria”‘ says Vandierendonck.
The global rise of bacteria that have become insensitive to antibiotics poses an increasing threat to public health. As conventional antibiotics are losing their effectiveness, there is an urgent need for new ways to combat bacterial infections. One of the bacteria identified by the World Health Organization as critical in the search for new treatments is Escherichia coli. While this gut bacterium is usually harmless, certain strains can cause severe infections such as intestinal diseases, urinary tract infections, and sepsis.
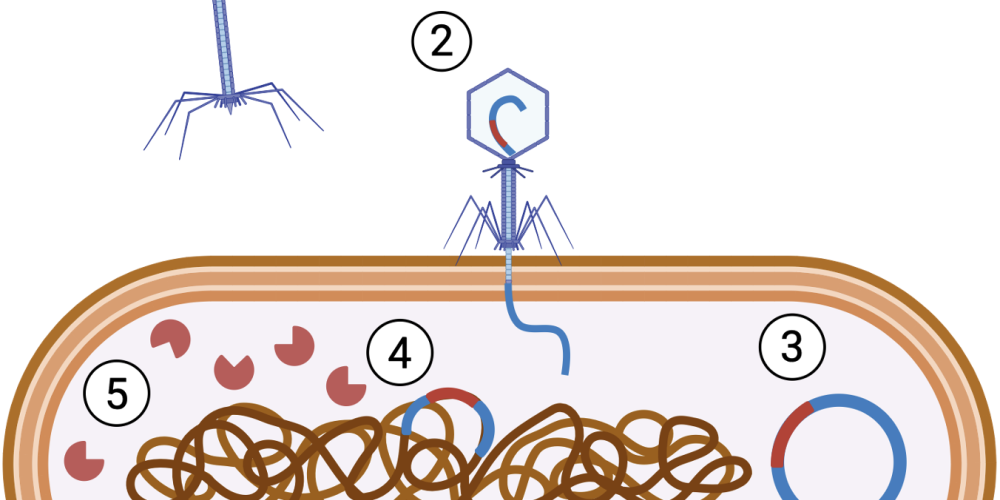
A bacteriophage can be compared to a kind of lunar lander that attaches itself to specific bacteria using bacterial receptors on the cell wall. Once attached, the phage injects its genetic material into the bacterial host. From that point, there are two possible pathways for reproduction. The phage may either exploit the bacterial “machinery” to produce new phages, which eventually burst the bacterial cell and kill it, or it may integrate its genetic material into the bacterial genome in a dormant state as a “temperate” phage. The former makes bacteriophages a promising and natural tool to selectively eliminate bacteria as an alternative to antibiotics. Temperate phages, by contrast, do not necessarily kill the bacterial cells, and this is where Vandierendonck’s research comes in.
In addition to phage therapy, she also explored the use of bacterial toxin–antitoxin systems, which consist of a toxin that induces cell death and an antitoxin that counteracts its effect. By combining phages and toxins, Jessie developed an innovative approach to target pathogenic E. coli bacteria. In her research, temperate phages were genetically engineered to deliver bacterial toxins to harmful bacteria. Once inside the bacterial cell, the toxin is expressed, effectively neutralising the bacterium.
“By using phages as a delivery vehicle for toxins, we can act with high precision, thereby reducing the risk of harming beneficial bacteria, unlike traditional antibiotics”, Vandierendonck explains.
Genetically manipulating toxin-bearing phages, however, proved challenging, as cloning toxin genes can halt the growth of the bacterial host cells. To overcome this obstacle, Jessie developed new cloning strategies in which toxin expression is tightly controlled at multiple levels. She also isolated phages from enterohaemorrhagic E. coli and characterised them both genetically and morphologically, including the identification of the bacterial receptors involved in infection.
As a proof of concept, she combined one of these phages with toxin genes and demonstrated in the laboratory that this recombinant phage could effectively kill the target bacterium via the toxin.
Jessie obtained her Master’s degree in Biology from VUB in 2020 and subsequently began her PhD within the Structural Biology Brussels research group, under the supervision of Prof. Dr. Ir. Remy Loris. During her doctoral studies, she published two first-author scientific papers, supervised Master’s students, and participated in several international conferences on bacteriophages.
“We hope this research represents a step forward in developing targeted, sustainable, and safe treatments against multidrug-resistant bacteria”, Jessie concludes.
More information:
📧 Jessie Vandierendonck – Jessie.Vandierendonck@vub.be
Isolation, characterization and genetic modification of toxin-bearing bacteriophages from enterohemorhaggic E.coli